|
In This Issue...
- Educational challenges today in the field of behavioral neuroscience
- Using a Multi-Conditioning System to perform an accurate and detailed behavioural assessment in rodents
- Morphine’s detrimental effects in spinal cord injury
- Contemporary challenges for early career researchers in behavioral neuroscience
- Looking for a new employment opportunity or struggling to find the right candidate? Meet the IBNS Career Center!
- Trending Science
Educational challenges today in the field of behavioral neuroscience
by Stacey J Sukoff Rizzo, Co-Chair of the IBNS Education & Training Committee
Associate Director of the Center for Biometric Analysis and Director of the Mouse Neurobehavioral Phenotyping Core, The Jackson Laboratory, California, USA.
 Rigor and reproducibility is a major issue for preclinical studies and in particular for techniques with inherent variability, such as behavioral assays. As behavioral neuroscience experts we often recognize that vitally important environmental and experimental details are frequently not described in publications, thereby not lending the data reproducible from the onset. While the NIH and several scientific journals have mandated guidelines and initiatives on rigor and reproducibility aimed at improving transparency in reporting, including more granular experimental details; there is substantial evidence of deficient training in the execution of scientifically rigorous behavioral experiments. Rigor and reproducibility is a major issue for preclinical studies and in particular for techniques with inherent variability, such as behavioral assays. As behavioral neuroscience experts we often recognize that vitally important environmental and experimental details are frequently not described in publications, thereby not lending the data reproducible from the onset. While the NIH and several scientific journals have mandated guidelines and initiatives on rigor and reproducibility aimed at improving transparency in reporting, including more granular experimental details; there is substantial evidence of deficient training in the execution of scientifically rigorous behavioral experiments.
Behavioral data generated by scientists with limited hands-on training in behavioral neuroscience are frequently conducted under “publish or perish” pressures to provide functionally translational and relevant endpoints for molecular findings. Those experiments are often conducted without proper controls or in sub-optimal behavioral testing facilities and the data are frequently misinterpreted or over-interpreted to fit the hypothesis. Furthermore, potential confounds such as the impact of changes in activity levels may not be reported or may be mentioned only briefly with insufficient emphasis.
Anecdotal information across laboratories that do not have behavioral neuroscience expertise has provided little confidence in the manner in which technicians and trainees are learning to conduct behavioral tests. A common practice in many laboratories seeking to establish a behavioral assay new to the lab is to conduct a literature search on a method of interest and to then perform the behavioral test in the fastest and most economical way possible.
Another common practice is the passing down of information or methods over time from transient students or post-docs in the laboratory to the subsequent personnel performing the assays. In such pass-down training, information may be inadvertently changed and granular details lost. For many scientific techniques in other fields, novices are prohibited from using equipment or running experiments without being appropriately trained or first demonstrating proficiency by reproducing data for a known standard.
Nevertheless, this does not seem to be the case for training in behavioral assays, particularly in molecular and cellular laboratories where behavioral testing is used minimally, often only as the final set of experiments. While these unfortunate practices suggest a lack of availability of expert hands-on and systematic training, this is not necessarily so, as many labs with established techniques welcome the opportunity for scientists and trainees to visit, observe, and in some cases conduct hands-on studies under the mentorship of the lab’s expert technical staff.
A new initiative this year for the E&T committee will be to identify labs and experts that are available to provide these types of hands-on behavioral training opportunities for visiting scientists. This type of training program is already in practice at The Jackson Laboratory’s (JAX) Mouse Neurobehavioral Phenotyping Facility and the committee will be reaching out to laboratories and behavioral testing core facilities with well-established expertise in specific techniques that share an interest in providing training to the greater research community. If your lab is interested in being a part of this initiative, please reach out to the E&T committee for more information.
Using a Multi-Conditioning System to perform an accurate and detailed behavioural assessment in rodents
by Nikolas Dietis, Guest Editor, IBNS Newsletter, September issue
Assistant Professor of Pharmacology, Medical School, University of Cyprus
Head of the UCY Experimental Pharmacology Laboratory
 It is widely accepted that performing an accurate and unbiased behavioural scoring, accomplishing a detailed and rigorous behavioural recording, as well as diminishing the effects of environmental influences (such as sound, scent, temperature, surrounding laboratory setting etc) on rodent behaviour during experimental testing, are challenging, demanding and time-consuming tasks (Balcombe, 2006). Most currently-used equipment and accepted set-ups for rodent behavioural screening share common limitations which mainly arise from the ineluctable interaction of the animal with the investigator prior and after screening, the inability to maintain stable and unaffected environmental factors during testing (such as sound, light, scent, temperature etc) and the limited ability of the equipment to offer customisation of every parameter within each protocol. These limitations have been extensively described in the literature (Steele et al, 2007; Richardson 2015) as factors that influence rodent behaviour. It is widely accepted that performing an accurate and unbiased behavioural scoring, accomplishing a detailed and rigorous behavioural recording, as well as diminishing the effects of environmental influences (such as sound, scent, temperature, surrounding laboratory setting etc) on rodent behaviour during experimental testing, are challenging, demanding and time-consuming tasks (Balcombe, 2006). Most currently-used equipment and accepted set-ups for rodent behavioural screening share common limitations which mainly arise from the ineluctable interaction of the animal with the investigator prior and after screening, the inability to maintain stable and unaffected environmental factors during testing (such as sound, light, scent, temperature etc) and the limited ability of the equipment to offer customisation of every parameter within each protocol. These limitations have been extensively described in the literature (Steele et al, 2007; Richardson 2015) as factors that influence rodent behaviour.
In an effort to minimize the above limitations (external environment, unbiased recording, limited customisation), our lab has been using a computer-controlled platform that we equipped with various instruments that offer detailed protocol tweaking, called a Multi-Conditioning System (MCS). The system operates in a closed environment using automated recordings & scorings to assess common rodent behaviours. We tested MCS in various experimental studies for depressive behaviour (Yin et al, 2016), PTSD studies, acute anxiety and memory studies, to evaluate its ability to overcome these limitations as described below.
The first advantage we observed in using the MCS platform is the capacity to perform behavioural testing in an insulated and ventilated ‘closed’ container that offers full control over external conditions. Its environment diminishes external influences during behavioural testing, such as noise and distant sounds, scents from the investigator or the surrounding equipment, variations in temperature and influence of lighting. The container is internally covered by an insulating material for complete insulation from external stimuli (sound, scent, light & temperature), therefore excluding the influence of these external factors during testing. Moreover, the containment of the testing area does not allow direct contact of the investigator with the animal during testing, while allowing the investigator or the team to interact and manage the experiment during testing without leaving the testing room.
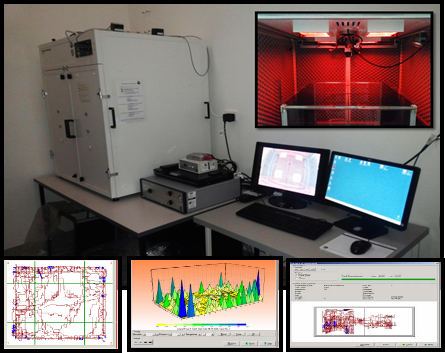
The second advantage we observed when using the MCS platform is the immense variety of parameter controls so that we can tailor each protocol in detail, providing both accuracy and “experimental precision” for protocol validation. We equipped the MCS with different modular components (arenas and detection systems) that were used in many different behavioural paradigms (fear conditioning, active & passive avoidance, place-preference, learned-helplessness, latent-inhibition, Light/Dark, locomotor activity and others) without the need to change the experimental conditions when alternating between paradigms. Animal positioning and movement detection is automatically recorded using a high-resolution 3D infrared light beam frame for fast movement detection in all three dimensions, while recording is performed using an infra-red camera to allow tests in absolute dark conditions. This has allowed us to be able to evaluate the ‘basal’ behaviour of an animal in close-to-natural conditions. White house lights of variable intensity with integrated covers for red light conditions reduce basal anxiety levels prior to recording, LEDs and a sound generator can present paradigm-controlled stimuli of variable intensity, a background white noise generator normalizes the internal sound levels, ultrasound ability is used for panic response tests, compartmentalised shock-grids are used for independent control of shocks and different surface structures are used in multiple arenas. This variation in automated equipment, along with dedicated software for automated recording, scoring, analysis and graphical representation, offers full-customisation ability that proved in our setting to be very important for the accuracy of our data.
References:
Balcombe JP, Laboratory environments and rodents' behavioural needs: a review. Lab Anim. 2006 Jul;40(3):217-35.
MCS, TSE Systems GmbH, Siemensstr. 21, 61352 Bad Homburg, Germany
Richardson C. A. The power of automated behavioural homecage technologies in characterizing disease progression in laboratory mice: A review. Appl AniM Beh Sci. 2015; 163: 19-27
Steele A, Walker S. J, King O. D and Lindquist S. The power of automated high-resolution behavior analysis revealed by its application to mouse models of Huntington's and prion diseases. Proc Natl Acad Sci U S A. 2007; 104(6): 1983–1988.
Yin X, Guven N, Dietis N. Stress-based animal models of depression: Do we actually know what we are doing? Brain Res. 2016 Dec 1;1652:30-42.
Morphine’s detrimental effects in spinal cord injury
by Michelle Hook, Assistant Professor, Department of Neuroscience and Experimental Therapeutics,
Texas A&M Health Science Center, USA ([email protected])
 In the critical first few days that follow spinal cord injury (SCI), many patients will experience distressing pain that arises from the trauma to the cord, the spinal nerves, the spinal fracture, or from concomitant injuries. Successful pain management in the emergency setting is essential as under-sedation may allow delirium to develop, and unmanaged acute pain is associated with an increased risk for the development of affective disorders (such as PTSD), cognitive impairments and chronic pain. Chronic pain is cited as one of the most significant consequences of SCI and one that patients most want cured. Opioids, including morphine, are among the most effective and frequently prescribed analgesics for management of this pain. In the critical first few days that follow spinal cord injury (SCI), many patients will experience distressing pain that arises from the trauma to the cord, the spinal nerves, the spinal fracture, or from concomitant injuries. Successful pain management in the emergency setting is essential as under-sedation may allow delirium to develop, and unmanaged acute pain is associated with an increased risk for the development of affective disorders (such as PTSD), cognitive impairments and chronic pain. Chronic pain is cited as one of the most significant consequences of SCI and one that patients most want cured. Opioids, including morphine, are among the most effective and frequently prescribed analgesics for management of this pain.
Unfortunately, however, we discovered that morphine has significant adverse effects on recovery of function in a rodent model of spinal cord injury. In the rodent model we found that a single administration of morphine (directly onto the spinal cord) on the day following SCI undermines locomotor recovery, increases long-term pain, and increases tissue loss at the injury site (Hook et al. 2009, 2011). Similarly, emulating the clinical administration of opioids, we found that intravenous (i.v) administration of morphine for the first 7 days of SCI is addictive, undermines locomotor recovery, and increases cell loss at the injury site (Woller et al. 2012; Hook et al. 2017). SCI per se decreases the expression of neuronal markers relative to uninjured controls. However, when i.v. morphine is added to the injury there are virtually no neurons, labeled with NeuN or neurofilament, across the rostral-caudal extent of the lesion. Glial expression is also decreased by 7 days of morphine administration. In SCI subjects treated with morphine, there are no GFAP+ astrocytes across the rostral-caudal extent of the lesion. The absence of GFAP is underscored by the increased expression in vehicle-treated SCI subjects.
These findings are alarming. It is tempting to suggest that morphine should not be used for pain management after SCI. However, for patients faced with a lifetime of intractable pain, simply removing morphine as a potential analgesic is not an option. We must understand the mechanisms that underlie the negative consequences of opioids. We have begun to address this in the rodent model. Using pharmacological manipulations we found that the detrimental effects of opioids are mediated by activation of the kappa-opioid receptor system. Administration of a kappa-opioid receptor agonist is sufficient to decrease locomotor recovery, and antagonizing the kappa receptor with nor-Binaltorphimine blocks the morphine-induced attenuation of functional recovery (Aceves et al 2016, 2017).
Our studies also suggest that microglia play a critical role in morphine’s adverse effects. Blocking microglial activation with minocycline prevents the decrease in locomotor recovery with morphine administration. Our current working hypothesis is that after SCI, activation of kappa opioid receptors on immune cells dramatically alters the consequences of opioids, producing a transition from neuron-mediated analgesia to glia-mediated excitotoxicity.
These data have significant implications for pain management, demonstrating the importance of the pathophysiological context in which the medication is applied. With few alternatives to opioids, we are not in the position to potentiate the risk of long-term pain after SCI and discard one of our most effective classes of analgesics. Instead, the goal of my laboratory is to identify the critical molecular changes underlying the adverse effects of opioids, and reduce the potential for harm with pharmaceutical intervention.
References:
Aceves, M., Mathai, B.B., Hook, M.A. (2016). Evaluation of the effects of specific opioid receptor agonists in a rodent model of spinal cord injury. Spinal Cord 54(10), 767-777.
Aceves, M., Bancroft, E.A., Aceves, A.R., Hook, M.A. (2017). Nor-Binaltorphimine Blocks the Adverse Effects of Morphine after Spinal Cord Injury. J Neurotrauma 34(6), 1164-1174.
Hook, M.A., Moreno, G., Woller, S., Puga, D., Hoy, K., Jr., Balden, R. and Grau, J.W. (2009). Intrathecal morphine attenuates recovery of function after a spinal cord injury. J Neurotrauma26, 741-752.
Hook, M.A., Washburn, S.N., Moreno, G., Woller, S.A., Puga, D., Lee, K.H. and Grau, J.W. (2011). An IL-1 receptor antagonist blocks a morphine-induced attenuation of locomotor recovery after spinal cord injury. Brain Behav Immun25, 349-359.
Woller, S.A., Moreno, G.L., Hart, N., Wellman, P.J., Grau, J.W. and Hook, M.A. (2012). Analgesia or addiction?: implications for morphine use after spinal cord injury. J Neurotrauma29, 1650-1662.
Hook, M.A., Woller, S.A., Bancroft, E., Aceves, M., Funk, M.K., Hartman, J., Garraway, S.M. (2017). Neurobiological Effects of Morphine after Spinal Cord Injury. J Neurotrauma. 34(3), 632-644.
Contemporary challenges for early career researchers in behavioral neuroscience
by Sarah Baracz, IBNS 2017 Early Career Award recipient
Associate Lecturer, Neuropsychopharmacology Laboratory, Department of Psychology, Macquarie University, Australia.

Early career researchers face many challenges in the current scientific climate. It is now much more competitive, with the number of awards, grants, scholarships, and publications required to get your foot in the door reaching improbable levels. This has left many early career researchers feeling dispirited about pursuing a career in science. In Australia, there are a limited number of postdoctoral positions available, and in the United States, they are typically poorly paid, making it difficult for young families to survive on such a low income. Applying for an early career research fellowship is equally grim, with low funding rates, and strategic planning required long before the submission deadline for a competitive application.
Grant applications submitted outside of the early career fellowship window (in Australia, applicants for government grants are considered’ early career’ when within 2 years of PhD completion) also have limited success as early career researchers are competing against more established scientists who have experience navigating the system. Coupled with the decline in government research funding being offered in many countries, this creates a bleak outcome.
Early career researchers now need to consider alternative options to traditional research career paths, as a large proportion of early career researchers will not find work in academia. In addition to difficulties acquiring independent research funding or a research position, early career researchers typically have limited or no access to resources that could help build their career. This support, which includes networking events, professional development workshops and career advice, is often provided by universities and institutions to their staff with permanent or fixed term positions. Because of this, many researchers who would benefit greatly from these resources, arguably at a time in their career when they need them most, are unable to access such support.
The development of one’s research identity can also be quite challenging for an early career researcher. As a graduate student, your research identity is very much linked to your PhD project and your mentor. Once completed, it is expected that your research identity grows and develops. This, however, can be quite difficult when your research interests do not completely align with your mentor’s, or you have been hired to manage a research project on a different topic or in a different area. It may be challenging to negotiate time to spend on developing and undertaking your own projects, or to learn a new technique or skill that will enhance future career prospects. And when your research interests do align, it can be difficult for the early career researcher to distinguish themselves from their mentor, and focus on their development as an intellectually independent researcher. Being an early career researcher can feel like limbo, where you are no longer a graduate student but not yet an independent researcher. This then makes the progression to becoming a career researcher seem even more blurry and distorted.
Now more than ever, it is important for early career researchers to establish and maintain relationships with supportive mentors who encourage and help you grow as a researcher, and are invested in your success. Seeking out, and actively engaging with societies, like IBNS, is also crucial as they provide ample support for their members and opportunities for networking and career development. Whilst there are many challenges that early career researchers currently face, being engaged in a supportive research environment and knowing that you are not alone in your struggles can help make the transition to research independence possible.
Looking for a new employment opportunity or struggling to find the right candidate? Meet the IBNS Career Center!
One of the biggest challenges for any international scientific society is to provide quality and informative support to its members, whether it is for a new employment opportunity or for finding the right candidate for a new position you opened. The IBNS excels in this area through its online Career Center portal (http://jobs.ibnsconnect.org) through which it provides the right tools for both, job seekers and employers.

The IBNS Career Center portal offers all the standard operational features, such as a thorough search engine by keyword and location, as well as extra services such as a free review of your resume for feedback and a job-posting service for employers. However, what makes the IBNS Career Center stand out in terms of support, is two additional quality features: resources for job seekers & access to a resume bank for employers.
In the Resources section you can have access to a number of articles with valuable tips in resume building, job seeking and communication, from experienced scientists in the field, not only for searching or applying for a position, but also for the interview process. There is also plenty of advice and tips for building your ‘brand’ and your social media presence, which will help you strengthen your image and moving your career to the direction you want.
 In the Resume Bank, potential employers have free access to a large bank of resumes and profiles, which you can customize by introducing filters that apply to your search and create lists of candidates that fulfill your own criteria. In the Resume Bank, potential employers have free access to a large bank of resumes and profiles, which you can customize by introducing filters that apply to your search and create lists of candidates that fulfill your own criteria.
Trending Science
In this column, we will share the latest research, interesting scientific articles and news you can use.
by Nikolas Dietis, Guest Editor, IBNS Newsletter, September issue
Assistant Professor of Pharmacology, Medical School, University of Cyprus
Head of the UCY Experimental Pharmacology Laboratory
Episodic memory formation and retrieval are processes regulated by specific neurons in the medial temporal lobe. New evidence about the important selective role of specific neurons in memory retrieval, has come to light. In a recent study published in Cell this August (Roy et al, 2017), Roy and colleagues show that a less-known subfield of hippocampal neurons, called the subiculum (Sub), and the circuit CA1 to dorsal-Sub (dSub) play this important selective role in retrieval of episodic memories.
The authors selectively targeted the dorsal Sub neurons by generating a transgenic mouse that expressed Cre recombinase under the control of fibronectin 1, which is exclusively expressed in dSub neurons. They then used optogenetic techniques to excite or inhibit the indirect pathway of episodic memory retrieval (from dCA1 to EC5) that passes through the dSub region, using a contextual fear-conditioning (CFC) paradigm and measuring the freezing behaviour of these mice after the recall, compared to non-conditioned animals. The results of the study concluded that the indirect pathway that runs via dSub is crucial in context-specific memory retrieval specifically. These data were backed up by neuronal activation studies using Fos-gene activation as biomarker, showing increased FOS expression in the dCA1 to dSub pathway neurons during memory retrieval, as well as by calcium imaging studies in dSub neurons that showed increased activation during retrieval rather than during training. This study by Roy et al provides the basis for further studies on the circuity and biology of these cells and their role in pathology and therapeutics.
Reference: Dheeraj S. Roy, Takashi Kitamura, Teruhiro Okuyama, Sachie K. Ogawa, Chen Sun, Yuichi Obata, Atsushi Yoshiki, Susumu Tonegawa. Distinct Neural Circuits for the Formation and Retrieval of Episodic Memories. Cell 2017; 170(5):1012.e19
Do you have an interesting hobby or member news to share?
Let us know at [email protected]
|